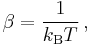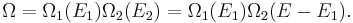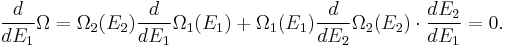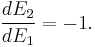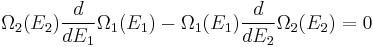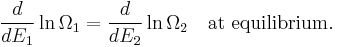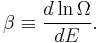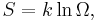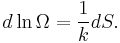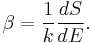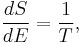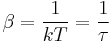Thermodynamic beta
In statistical mechanics, the thermodynamic beta is a physical quantity related to the thermodynamic temperature 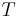 of a system. It can be calculated from formula
of a system. It can be calculated from formula
where 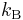 is the Boltzmann constant. The thermodynamic beta can be viewed as a connection between the statistical interpretation of a physical system and thermodynamics. It is sometimes considered a more fundamental quantity than temperature.
is the Boltzmann constant. The thermodynamic beta can be viewed as a connection between the statistical interpretation of a physical system and thermodynamics. It is sometimes considered a more fundamental quantity than temperature.
Contents |
Details
Statistical interpretation
From the statistical point of view, β is a numerical quantity relating two macroscopic systems in equilibrium. The exact formulation is as follows. Consider two systems, 1 and 2, in thermal contact, with respective energies E1 and E2. We assume E1 + E2 = some constant E. The number of microstates of each system will be denoted by Ω1 and Ω2. Under our assumptions Ωi depends only on Ei. Thus the number of microstates for the combined system is
We will derive β from the following fundamental assumption:
- When the combined system reaches equilibrium, the number Ω is maximized.
(In other words, the system naturally seeks the maximum number of microstates.) Therefore, at equilibrium,
But E1 + E2 = E implies
So
i.e.
The above relation motivates the definition of β:
Connection with thermodynamic view
On the other hand, when two systems are in equilibrium, they have the same thermodynamic temperature T. Thus intuitively one would expect that β be related to T in some way. This link is provided by the formula
where k is the Boltzmann constant. So
Substituting into the definition of β gives
Comparing with the thermodynamic formula
we have
where 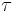 is sometimes called the fundamental temperature of the system with units of energy.
is sometimes called the fundamental temperature of the system with units of energy.